Device Pipeline and Approvals
Latest News
Latest Videos
CME Content
More News

System is designed to provide fully automated, near real-time ECG interpretation at enterprise scale.

Edwards Lifesciences device expands options for patients with severe mitral regurgitation who are not candidates for surgery
Shape Memory Medical's IMPEDE Embolization Plug gains Class III certification in Europe, enhancing access and confidence in advanced endovascular therapies.

SleepRes is introducing the Kricket PAP device, which achieved FDA clearance and is a solution for sleep apnea that enhances comfort and adherence through adaptive pressure technology.

Device addresses PCOS-related infertility for those who do not respond to standard drug therapies

BlueWind Medical's Revi wearable for urinary incontinence offers effective, user-friendly therapy that boosts patient satisfaction and long-term relief.
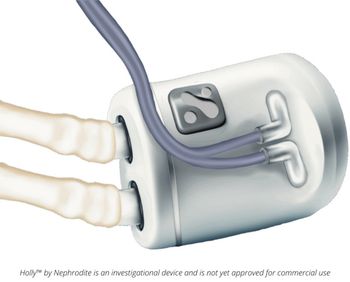
Nephrodite's Holly system advances kidney care with an implantable continuous dialysis solution, enhancing patient freedom and quality of life.

Flow Neuroscience's FDA-approved device offers an at-home treatment for depression, providing a non-drug option with proven effectiveness and minimal side effects.

Bendit Technologies launches the Bendit17 Microcatheter, enhancing precision in vascular procedures with FDA clearance for U.S. market entry.
Blood-based diagnostic aims to reduce unnecessary prostate biopsies and improve cancer risk assessment.

GE Healthcare launches Pristina Recon DL, a new AI-driven mammography technology enhancing image quality and supporting early breast cancer detection.

Transmural Systems launches TELLTALE, an innovative electrosurgical guidewire, enhancing safety in transcatheter aortic valve replacement procedures.

Dexcom Smart Basal optimizes insulin dosing for Type 2 diabetes, integrating CGM data for personalized recommendations and enhancing patient care efficiency.

BrainsWay's FDA-approved Deep TMS system offers new hope for adolescents with depression, enhancing access to effective noninvasive treatment options.
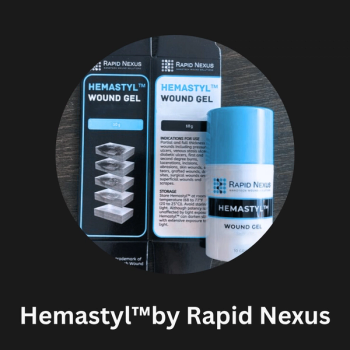
FDA 510(k) clearance for its Hemastyl gel device marks the first technology that has treated the tissue surrounding chronic wounds and prevented numerous amputations.
Hubly Surgical's FDA-cleared auto-stop drill enhances spinal surgery safety, preventing injury with innovative technology for precise, efficient procedures.

Augmedics unveiled the X2 augmented reality surgical headset, which enhances precision and comfort in spinal surgeries with advanced imaging and ergonomic design.
ZAP Surgical's new Axon system advances brain radiosurgery with FDA and CE approvals, enhancing precision and efficiency in treatment planning.
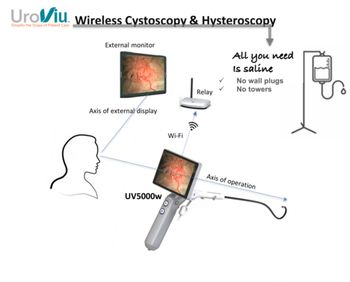
UroViu's CE-certified portable endoscopy device offer procedures with wireless connectivity, enhancing efficiency and patient care in urology and urogynecology.
Sirona Medical launches its FDA-cleared Advanced Imaging Suite, boosting radiology with cloud-native PET-CT capabilities for enhanced diagnostic flexibility.

GeneDx's genomic tests gain FDA Breakthrough Device Designation, enhancing diagnosis of life-threatening diseases through advanced whole genome and exome testing.

OcuMet Beacon enhances early detection and management of retinal diseases
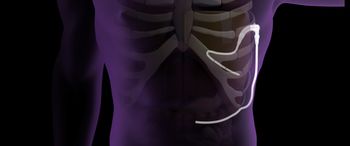
New CMS reimbursement code supports wider hospital adoption of Pleural Dynamics’ fully implantable ACES device for chronic pleural effusion treatment.
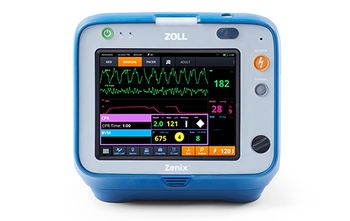
Asahi Kasei unveils the ZOLL Zenix defibrillator, enhancing emergency care with advanced technology and real-time guidance for health care professionals.