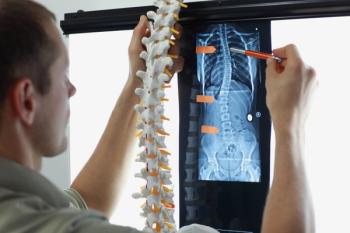
Ruthless Spine gains FDA clearance for its NavJam device, advancing spinal navigation with cost-effective, portable solutions for accurate screw placement.

Ruthless Spine gains FDA clearance for its NavJam device, advancing spinal navigation with cost-effective, portable solutions for accurate screw placement.
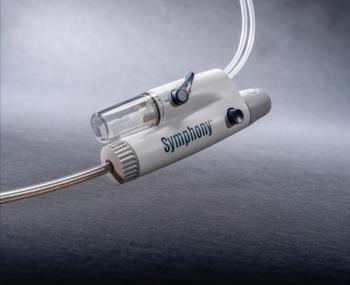
Imperative Care's Symphony Thrombectomy System gains FDA clearance, advancing pulmonary embolism treatment with enhanced control and faster recovery options.
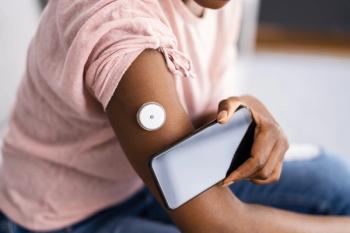
Glucose biosensor combined with artificial intelligence-powered platform provides real-time insights into how food, exercise, stress, and sleep affect the body.
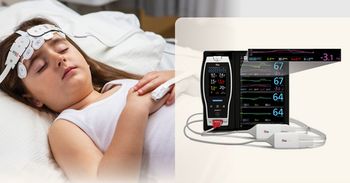
Masimo's O3 Regional Oximetry gains FDA clearance, enhancing tissue oxygen monitoring for improved patient care in critical settings.
ArteraAI Prostate advances prostate cancer care with FDA authorization, offering AI-driven insights for personalized treatment and improved diagnostic efficiency.

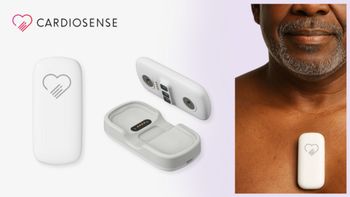
Cardiosense's CardioTag gains FDA clearance, advancing cardiac monitoring with wearable technology for personalized, noninvasive heart health insights.

Takeda introduces HyHub and HyHub Duo, innovative devices simplifying at-home administration of HYQVIA, enhancing patient experience in immune therapy.

Caranx Medical's AI software advances TAVI procedures, enhancing precision and accessibility for heart valve surgeries, improving patient outcomes.
Bruin Biometrics CEO Martin Burns discusses the "formulas" that guided the company through the challenges of bringing a device to market.

ArteraAI Prostate advances prostate cancer treatment with AI-driven insights, enhancing risk assessment and personalizing care for better patient outcomes.

In a wide-ranging interview, Martin Burns discusses Bruin Biometrics’ Provizio SEM Scanner, why diagnostic and prevention tech innovation is underfunded and the challenges and opportunities facing the MedTech industry.

System integrates handheld robotics, real-time ultrasound, and advanced software to help clinicians consistently and accurately place needles during procedures like organ access, biopsies, vascular access, and therapy delivery.

In a wide-ranging interview, Martin Burns discusses Bruin Biometrics’ Provizio SEM Scanner, why diagnostic and prevention tech innovation is underfunded and the challenges and opportunities facing the MedTech industry.

Wristband delivers personalized, adaptive stimulation throughout the day without the need for surgery or drugs

Solution is for patients suffering from severe ankle joint damage due to rheumatoid, post-traumatic, or degenerative arthritis
The FDA’s IDE approval allows Presidio to initiate a randomized, controlled trial in the United States and Australia to evaluate the safety and efficacy of its ULF neuromodulation technology
The novel test uses a machine learning algorithm and multiple cardiac protein markers to detect obstructive coronary artery disease through a simple blood draw.
Company eyes FDA clearance for expanding use of real-time navigation technology

Guardant Health's Shield multi-cancer screening test detects multiple cancer types with a single blood draw, promising early diagnosis for various cancers.

Reflow Medical's Spur Stent System gains FDA clearance, offering treatment options for chronic limb-threatening ischemia with its retrievable design.
Company expects to submit to FDA in 2026

Atia Vision's OmniVu lens system promises a breakthrough in cataract surgery with enhanced vision restoration.
Fujirebio Diagnostics’ test, which received 510k clearance Friday, will “assist physicians and patients to obtain an AD diagnosis in early stages of the disease.”

Osteoboost introduces a groundbreaking FDA-cleared device for osteoporosis, offering innovative, non-invasive therapy to enhance bone health and prevent fractures.