
Medical Device & Technology
Latest News
Latest Videos

CME Content
More News
New CMS reimbursement code supports wider hospital adoption of Pleural Dynamics’ fully implantable ACES device for chronic pleural effusion treatment.

A year after its landmark artificial intelligence summit, JAMA says health systems are deploying unproven algorithms with little evidence they improve outcomes — or even do no harm.
AngioDynamics' NanoKnife System advances prostate cancer treatment, offering a minimally invasive option that preserves healthy tissue and enhances patient outcomes.
Asahi Kasei unveils the ZOLL Zenix defibrillator, enhancing emergency care with advanced technology and real-time guidance for health care professionals.

Horizon Surgical Systems Polaris platform enhances precision and safety in ophthalmic procedures through AI integration.

Four other companies garner $25,000 each as finalists
Study addresses equity concerns in critical care monitoring in pulse oximetry.

The Australian startup aims to scale ambient AI tool that automates administrative work in health care systems worldwide.

Basil Systems boosts medical device safety with AI, transforming post-market surveillance into proactive risk detection and enhancing compliance.
Nihon Kohden's AlarmSense tackles alarm management, reducing alarm fatigue and enhancing patient safety while supporting clinician well-being in health care settings.

AngioSafe's Santreva-ATK catheter advances endovascular treatment for chronic total occlusions, enhancing patient care with innovative, wire-free technology.

Freudenberg Medical opens a $25 million facility in Costa Rica, enhancing MedTech production and showcasing advanced technologies for efficient manufacturing.

Medical consumables such as syringes, sutures, catheters and gauze, as well as personal protective equipment like gloves and masks, are all receiving scrutiny.

Neural Sleeve 2 uses AI-driven MultiStim technology for muscle activation and spasm relaxation, aiding conditions like multiple sclerosis and stroke.

New patient monitoring device doesn’t require wearables or cameras

Glooko enhances diabetes care by acquiring Monarch Medical, expanding its solutions from outpatient to inpatient glycemic management for improved patient outcomes.

Morphic Medical launches RESET, a non-surgical treatment for obesity and diabetes, offering safe, effective weight loss and blood sugar control.

Medtronic's Altaviva device advances urinary incontinence treatment with its minimally invasive tibial neuromodulation technology.
Ruthless Spine gains FDA clearance for its NavJam device, advancing spinal navigation with cost-effective, portable solutions for accurate screw placement.
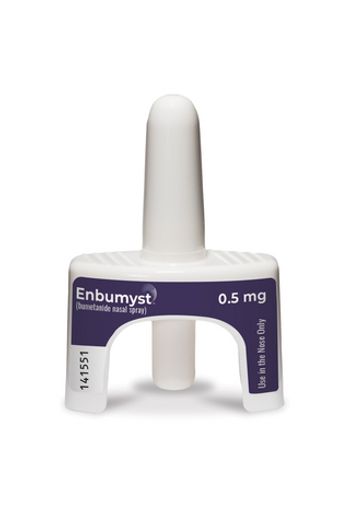
Unidose Liquid System will serve as the delivery device for Enbumyst, a newly approved nasal spray formulation of the loop diuretic bumetanide

Arlington Capital’s TEAM Technologies acquires TAG3 Engineering to boost its capabilities in the medical device space
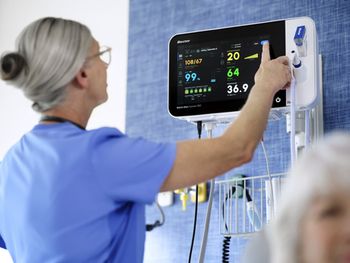
Baxter's Welch Allyn Connex 360 enhances patient monitoring with rapid vital sign capture, improved data security, and seamless integration for health care teams.















