
GeneDx's genomic tests gain FDA Breakthrough Device Designation, enhancing diagnosis of life-threatening diseases through advanced whole genome and exome testing.
Jupiter Endovascular reports positive first-in-human study results for pulmonary embolism device

Next generation chronic rhinitis treatment device gains expanded Medicare coverage

GeneDx's genomic tests gain FDA Breakthrough Device Designation, enhancing diagnosis of life-threatening diseases through advanced whole genome and exome testing.

OcuMet Beacon enhances early detection and management of retinal diseases
New CMS reimbursement code supports wider hospital adoption of Pleural Dynamics’ fully implantable ACES device for chronic pleural effusion treatment.
AngioDynamics' NanoKnife System advances prostate cancer treatment, offering a minimally invasive option that preserves healthy tissue and enhances patient outcomes.

Horizon Surgical Systems Polaris platform enhances precision and safety in ophthalmic procedures through AI integration.

Four other companies garner $25,000 each as finalists
Study addresses equity concerns in critical care monitoring in pulse oximetry.

The Australian startup aims to scale ambient AI tool that automates administrative work in health care systems worldwide.

Basil Systems boosts medical device safety with AI, transforming post-market surveillance into proactive risk detection and enhancing compliance.
Nihon Kohden's AlarmSense tackles alarm management, reducing alarm fatigue and enhancing patient safety while supporting clinician well-being in health care settings.
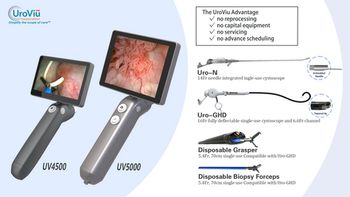
AngioSafe's Santreva-ATK catheter advances endovascular treatment for chronic total occlusions, enhancing patient care with innovative, wire-free technology.

New patient monitoring device doesn’t require wearables or cameras

Morphic Medical launches RESET, a non-surgical treatment for obesity and diabetes, offering safe, effective weight loss and blood sugar control.

Medtronic's Altaviva device advances urinary incontinence treatment with its minimally invasive tibial neuromodulation technology.
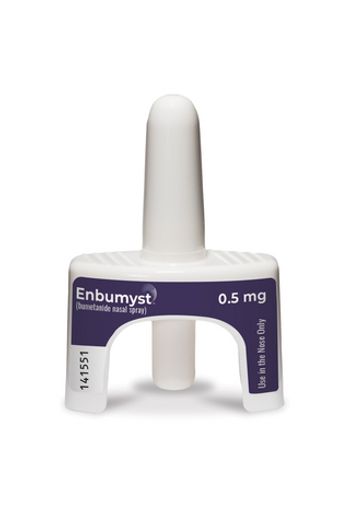
Unidose Liquid System will serve as the delivery device for Enbumyst, a newly approved nasal spray formulation of the loop diuretic bumetanide
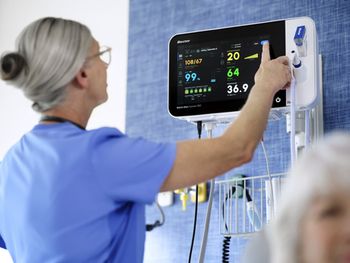
Baxter's Welch Allyn Connex 360 enhances patient monitoring with rapid vital sign capture, improved data security, and seamless integration for health care teams.

ShiraTronics unveiled an implantable device that offers sustained migraine relief, reducing headache days and improving quality of life for patients.

Deal aims to broaden device integration, speed up adoption of next-gen monitoring tools.

Apple Watch Series 11 introduces notifications for possible chronic high blood pressure. The company projects to catch more than one million previously undiagnosed cases of hypertension within a year of release.
FDA greenlights NANOCLAMP AF trial of nanosecond pulsed field ablation technology, enrolling patients across the U.S. and Europe.

Asahi Kasei unveils camera-free radar modules for health monitoring, enhancing privacy and safety of patients at home and in health facilities.
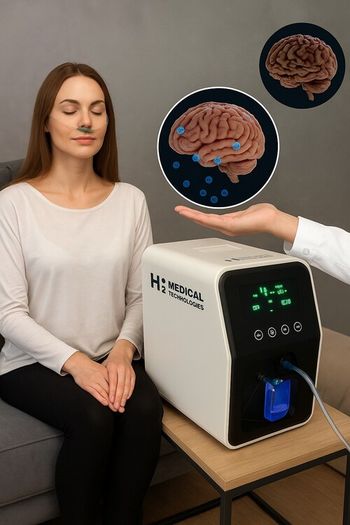
The device is designed to both prevent and treat the neurodegenerative condition, which affects more than 6.5 million Americans and 50 million people worldwide
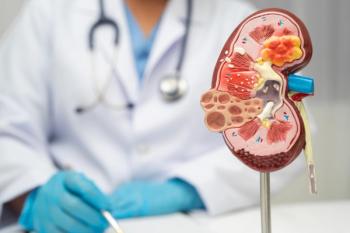
The partnership aims to improve early detection and management of CKD in high-risk and underserved populations by integrating Carna Health’s digital platform with Siemens Healthineers’ global diagnostics infrastructure.