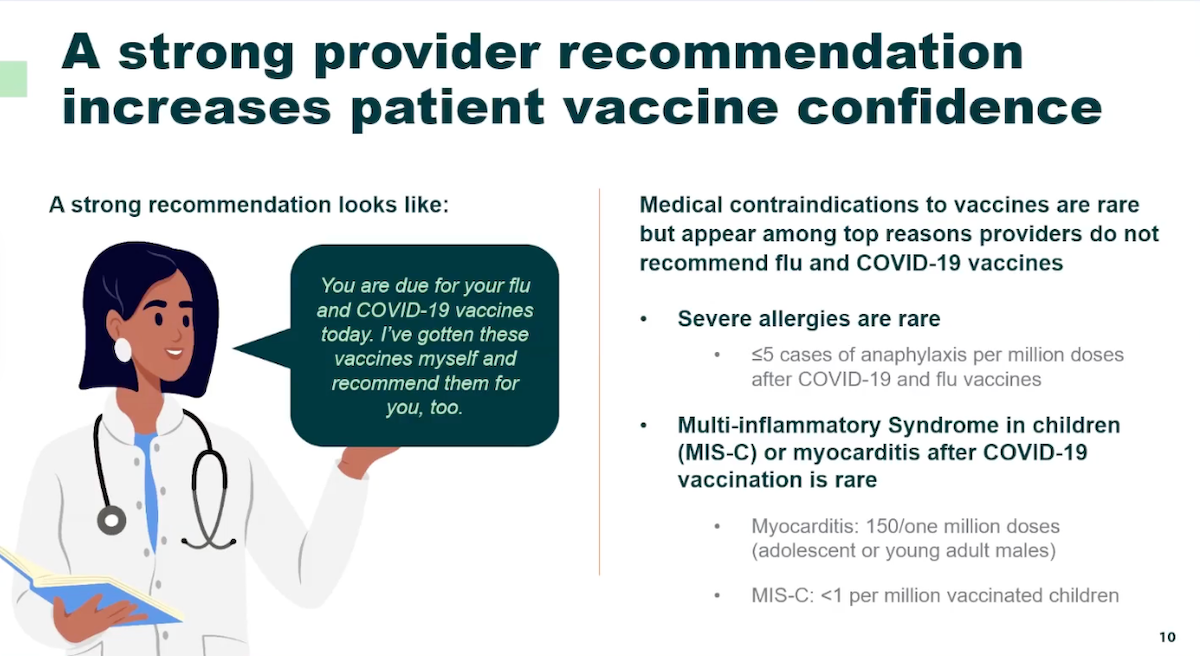
Primary care physicians are essential in combating the HIV epidemic by normalizing HIV testing and prescribing preventive medications. This approach not only enhances health equity and literacy among particularly underserved populations, but also expands their practice by attracting and retaining a broader patient base.