
Two new projects from IBM Research will put artificial intelligence to work in teaching medical students and making sense of electronic medical records.

Two new projects from IBM Research will put artificial intelligence to work in teaching medical students and making sense of electronic medical records.

Despite progress to resolve the current fiscal crisis, drug and medical device companies poised to seek regulatory approval from the FDA are still waiting for action as the agency puts new reviews on hold.

A divided U.S. Supreme Court has ruled that police may take and analyze DNA samples from anyone arrested for a "serious offense" as part of the gathering of forensic evidence.

Efforts to extend the reach of life-saving vaccines will cost billions and pose tough pricing challenges for innovators. The current strategy of differential pricing is proving problematic.
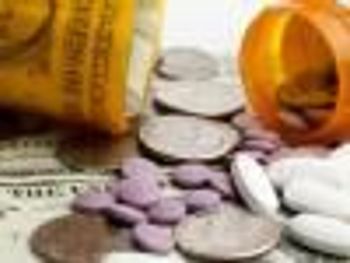
A group of more than 100 cancer experts concerned about the high prices for therapies for chronic myeloid leukemia and other cancers is appealing to drugmakers to lower prices on costly drugs to allow more patients to afford them.

Americans skipped doses, delayed filling prescriptions, and sought lower-cost medications in a battle to minimize the $45 billion out-of-pocket in expenses they incurred buying retail prescription drugs in 2011.

The percentage of first-year medical residents who reported concerns about making a serious medical error has risen with growing workloads, calling into question the benefits of a mandated cutback in their work hours, according to a new study.

Two U.S. senators have reintroduced legislation targeting "pay-for-delay" agreements, in which generic drugmakers agree to postpone competing with branded drugs in exchange for payment.

Researchers creating the world's most detailed model of the human brain to speed understanding of cognition and disease won a 10-year $1.4 billion award from the European Commission to support its work.

Despite signs of promise, researchers say more work is needed to establish the usefulness of mobile phones in care, although text messaging showed promise in increasing adherence.

Using generic HIV medications in place of brand-name drugs could save nearly $1 billion a year; but moving patients from a single-pill branded drug regimen to a multi-pill course of generics may diminish the effectiveness of HIV treatment.

The FDA ended 2012 with a bang, approving eight new drugs in December and pushing the year's total to 39, its highest level since 1996 when the agency cleared a backlog of applications.

Life sciences research and development investment will rise in 2013, even as some of the industry's biggest players pare their R&D spending, according to a forecast.

The U.S. Supreme Court will decide the legality of "pay-for-delay" agreements, in which generic drugmakers agree to postpone competing with branded drugs in exchange for payment.

A proposed act in Congress would create new a Office of Wireless Health Technology within the FDA to advance the health information technology sector with financial incentives, grants and workforce development programs.

The top pharmaceutical companies have improved access to their products in developing countries, developing more products for more diseases that disproportionately impact the world's poor and providing tiered pricing schemes.

Annual health checks neither reduce overall mortality nor the risk of dying from cardiovascular disease or cancer. However, they do increase the number of new diagnoses, which might do more harm than good.

The FDA won a small victory in a legal dispute with the National Whistleblower Center over the agency's right to withhold documents relating to its medical device review process.
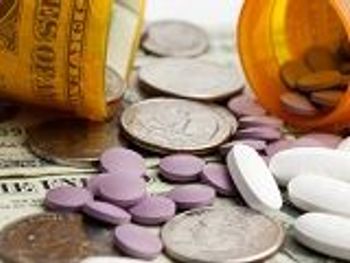
A new study shows that policies to bolster patients' adherence to prescribed therapies could trim $269 billion from health care costs annually.

Drugmakers' pipeline success rates across every phase of development have been slowly worsening or, at best, staying flat. Lagging R&D is one of the industry's biggest problems.

The U.S. Food and Drug Administration has proposed a rule for adding unique identifiers to most medical devices distributed in the United States.

The House unanimously approved a bill that would provide the FDA with $6.4 billion in fees from drugmakers over the next five years.
A new set of computer models designed to predict the side effects of current and experimental drugs holds the potential to improve drug development and patient treatment.

Federal regulators struggling to catch up with global supply chains are working to strengthen ties to their foreign counterparts in China and beyond to better protect public health at home.

A new study questions the utility of Hospital Compare in prompting hospitals to improve quality of care.

New website and free app from Kaiser Permanente put a patient's lab results and prescription refill requests on mobile phones.

Despite regulatory and reimbursement questions, big opportunities remain for health-related apps. It's expected that revenue from mHealth apps will build strongly in the U.S. and even in Africa and Southeast Asia.

A year-end tally of U.S. prescription drug shortages shows a 27% jump in the number of recorded shortfalls, but a new FDA rule and proposed legislation offer some hope for progress.
Whistleblowers are helping the Department of Justice expand its pursuit of false claims and police the pharmaceutical industry.

Digital health pioneers in California and Missouri are investing millions of dollars in new technologies to enable doctors, nurses and other health care workers help patients in remote areas as a new law lays the groundwork for telehealth adoption.

Published: January 23rd 2012 | Updated:

Published: January 30th 2013 | Updated:
Published: February 12th 2013 | Updated:

Published: April 30th 2013 | Updated:
Published: January 9th 2012 | Updated:

Published: June 29th 2011 | Updated: